Clinical Development Plan Template
Clinical Development Plan Template - Find out the differences between pilot, pivotal, and pmcf studies and. A clinical development plan (cdp) is key to bringing an investigational medicinal product (imp) to market, as it describes the potential risks and opportunities surrounding a clinical program. The professional development plan template is but one of over 100 free project management templates for excel and word that can be downloaded from our site. Alignment of key milestones into a single timeline for each phase of the project. Developing a comprehensive clinical trial protocol. Some manufacturers may need more clarity,. “annex xiv of the medical devices regulation (eu) 2017/745 (mdr), section 1(a), 8th indent, requires a clinical development plan (cdp). This clinical development plan includes: They help ensure that the data collected is high quality and, thus, can be used for regulatory. Assignment of a unique milestone shape to each type of milestone. Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020. Assignment of a unique milestone shape to each type of milestone. Some manufacturers may need more clarity,. “annex xiv of the medical devices regulation (eu) 2017/745 (mdr), section 1(a), 8th indent, requires a clinical development plan (cdp). This clinical development plan includes: You can use these as samples or even as a starting point for your own paper. This guide covers the key components, such as. Goals / definition clinical safety data and rationale for phase 2 dose selection. 의료기기의 안전성과 성능을 입증하기 위해 체계적인 임상 개발 계획(clinical development plan, cdp)의 수립은 필수적입니다. Alignment of key milestones into a single timeline for each phase of the project. A clinical development plan (cdp) is key to bringing an investigational medicinal product (imp) to market, as it describes the potential risks and opportunities surrounding a clinical program. Slideteam's commitment to excellence is evident in the superior quality of their. The clinical development plan is the blueprint of the entire clinical research strategy of a drug which defines the critical. It outlines the steps from early stages to approval and. Detailed plan for phase 1 and high level plan for phase 2 and 3 in place. Learn how to create a comprehensive and strategic clinical development plan for your new pharmaceutical or biotechnology product. Download the available cdp template to facilitate the multidisciplinary development of a clinical program that is. (*clinical development plan is initiated prior to the fih gate. It ensures consistency across clinical trial. Slideteam's commitment to excellence is evident in the superior quality of their. The professional development plan template is but one of over 100 free project management templates for excel and word that can be downloaded from our site. They help ensure that the data. This clinical development plan includes: It outlines the steps from early stages to approval and. Find out the differences between pilot, pivotal, and pmcf studies and. This guide covers the key components, such as. Some manufacturers may need more clarity,. “annex xiv of the medical devices regulation (eu) 2017/745 (mdr), section 1(a), 8th indent, requires a clinical development plan (cdp). Developing a comprehensive clinical trial protocol. Find out the differences between pilot, pivotal, and pmcf studies and. The protocol is the backbone of your clinical trial, detailing every step of the study. The professional development plan template is but one. Ppt templates are a tool to ensure that clinical studies are conducted appropriately. Alignment of key milestones into a single timeline for each phase of the project. Developing a comprehensive clinical trial protocol. A comprehensive clinical development plan (cdp) is crucial for the successful advancement of medical devices from concept to market. It ensures consistency across clinical trial. It ensures consistency across clinical trial. Ppt templates are a tool to ensure that clinical studies are conducted appropriately. Learn how to create a comprehensive and strategic clinical development plan for your new pharmaceutical or biotechnology product. You can go over several clinical development plans on this page before you start drafting your own. Slideteam's commitment to excellence is evident. Alignment of key milestones into a single timeline for each phase of the project. You can use these as samples or even as a starting point for your own paper. “annex xiv of the medical devices regulation (eu) 2017/745 (mdr), section 1(a), 8th indent, requires a clinical development plan (cdp). It ensures consistency across clinical trial. This clinical development plan. You can go over several clinical development plans on this page before you start drafting your own. Alignment of key milestones into a single timeline for each phase of the project. This guide covers the key components, such as. Ppt templates are a tool to ensure that clinical studies are conducted appropriately. Customize this template to create a document that. Goals / definition clinical safety data and rationale for phase 2 dose selection. “annex xiv of the medical devices regulation (eu) 2017/745 (mdr), section 1(a), 8th indent, requires a clinical development plan (cdp). Developing a comprehensive clinical trial protocol. Detailed plan for phase 1 and high level plan for phase 2 and 3 in place. 의료기기의 안전성과 성능을 입증하기 위해. A comprehensive clinical development plan (cdp) is crucial for the successful advancement of medical devices from concept to market. Download the available cdp template to facilitate the multidisciplinary development of a clinical program that is robust, feasible and aligned with the patient and payer value proposition. This clinical development plan includes: Some manufacturers may need more clarity,. 의료기기의 안전성과 성능을 입증하기 위해 체계적인 임상 개발 계획(clinical development plan, cdp)의 수립은 필수적입니다. It outlines the steps from early stages to approval and. Slideteam's commitment to excellence is evident in the superior quality of their. “annex xiv of the medical devices regulation (eu) 2017/745 (mdr), section 1(a), 8th indent, requires a clinical development plan (cdp). Find out the differences between pilot, pivotal, and pmcf studies and. Customize this template to create a document that describes the strategy and techniques for developing a new medical treatment. Learn how to create a comprehensive and strategic clinical development plan for your new pharmaceutical or biotechnology product. A clinical development plan (cdp) is key to bringing an investigational medicinal product (imp) to market, as it describes the potential risks and opportunities surrounding a clinical program. It ensures consistency across clinical trial. The protocol is the backbone of your clinical trial, detailing every step of the study. Goals / definition clinical safety data and rationale for phase 2 dose selection. Alignment of key milestones into a single timeline for each phase of the project.Clinical Development Plan Template
Clinical Development Plan Template in Google Docs, Pages, Word
Top 20 PowerPoint Templates to Create a Clinical Development Plan
Clinical Development Plan Template Fda prntbl.concejomunicipaldechinu
Clinical Development Plan Template Google Docs, Word, Apple Pages
Top 20 PowerPoint Templates to Create a Clinical Development Plan
Clinical Development Plan Template
Clinical Development Plan Template Peterainsworth
30 Clinical Development Plan Template Hamiltonplastering
Clinical Development Plan Template in Google Docs, Pages, Word
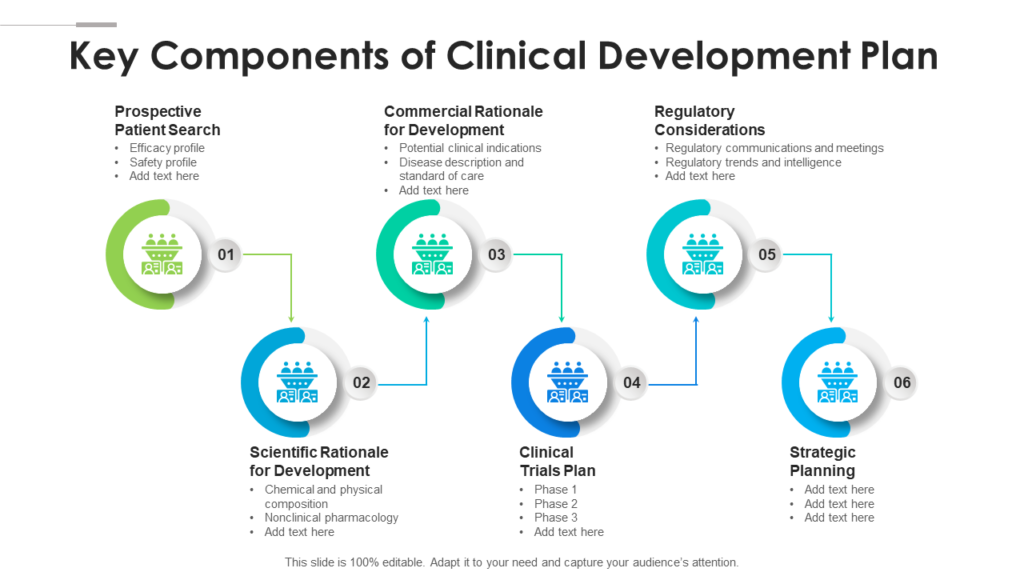
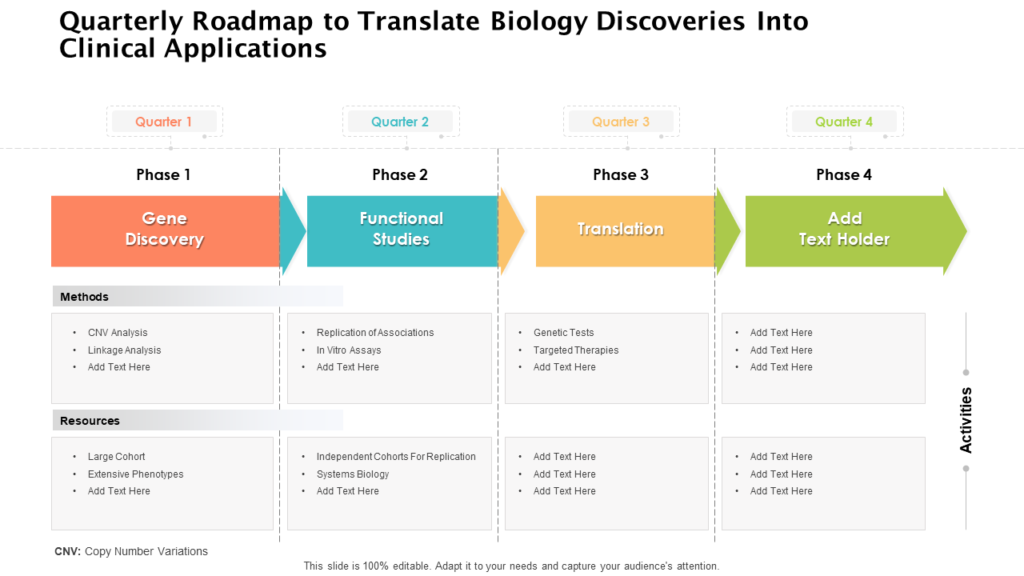
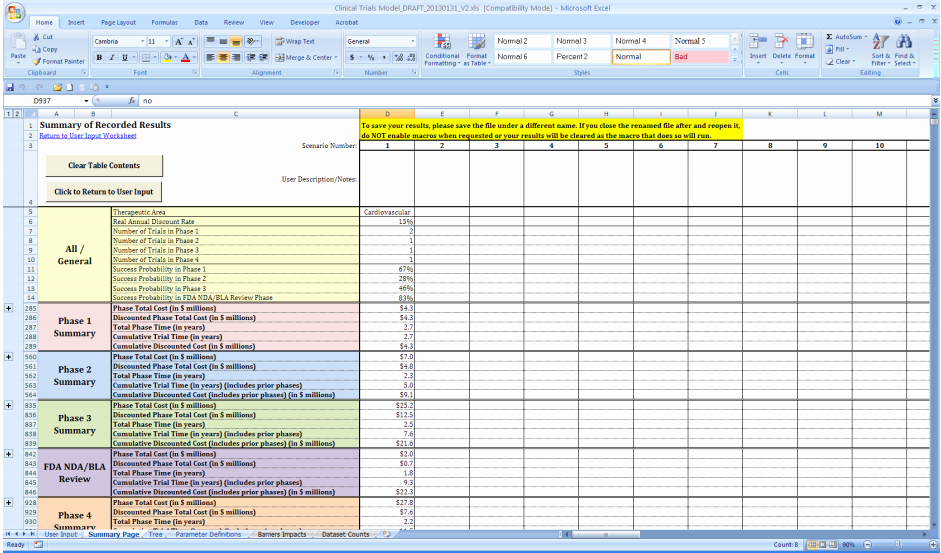
The Clinical Development Plan Is The Blueprint Of The Entire Clinical Research Strategy Of A Drug Which Defines The Critical Path For The Clinical Program Including Development.
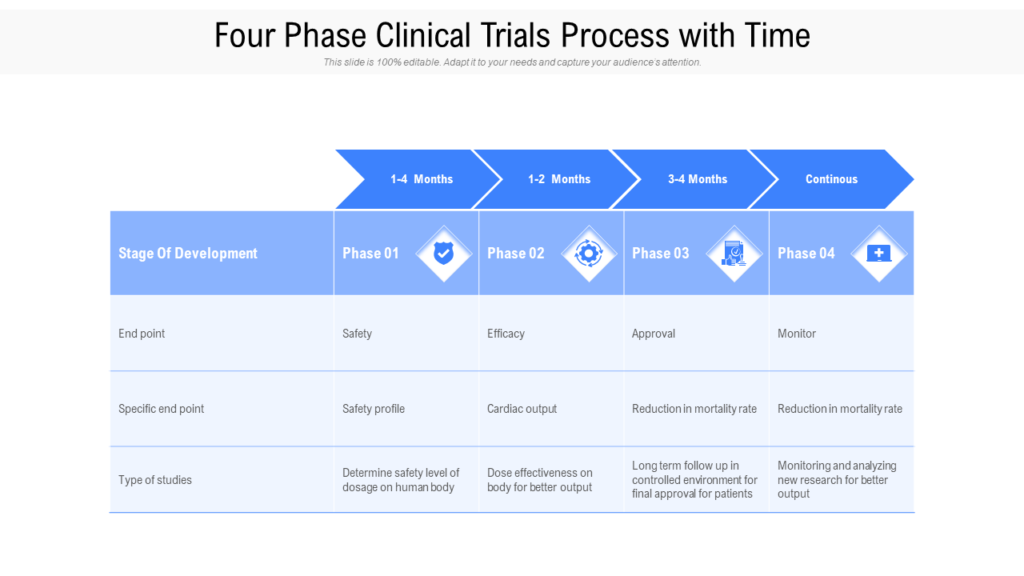
Detailed Plan For Phase 1 And High Level Plan For Phase 2 And 3 In Place.
This Guide Covers The Key Components, Such As.
Developing A Comprehensive Clinical Trial Protocol.
Related Post: