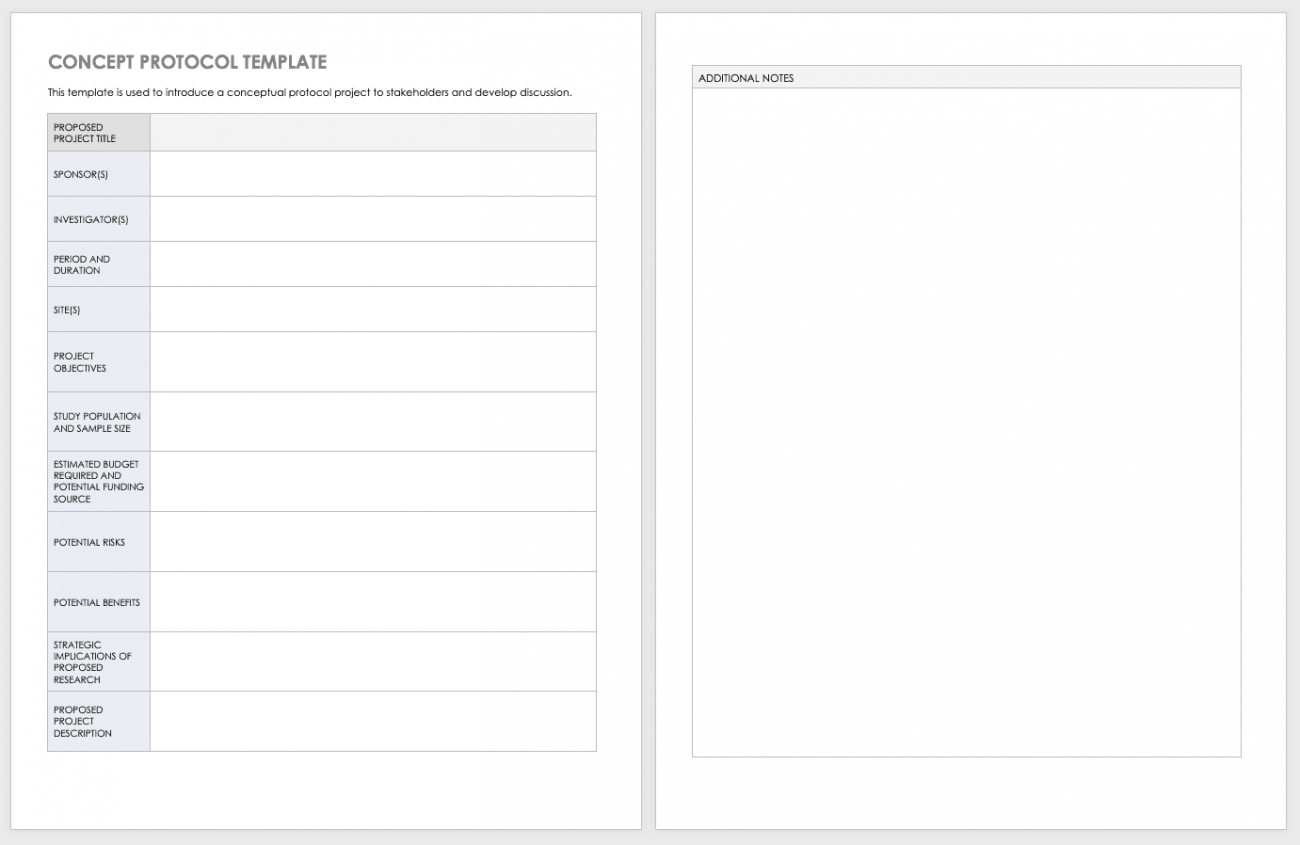
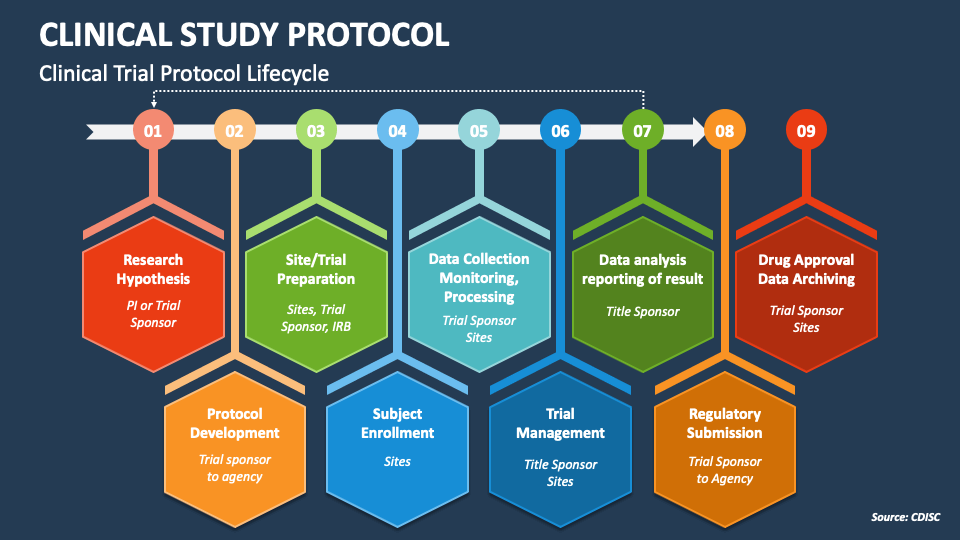
Clinical Study Protocol Template
Clinical Study Protocol Template - Trials is experimenting with a new way of structuring study protocols for randomised trials. In this blog, you have access to the links to the clinical trial protocol template from several regulatory bodies. The proposed guidance document includes a standardized template for manufacturers or other sponsors to use when developing ced study protocols using rwd. The goal of this template is to. The simple innovation is to include all 51 spirit headings and item identifiers within the protocol. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human participants. This report presents the explanation and elaboration paper for the consort (consolidated standards of reporting trials) 2010 and spirit (standard protocol items:. Section headings and template text formatted in regular type should be included in your protocol document as provided in the template. Developing a comprehensive clinical trial protocol. This report presents the explanation and elaboration paper for the consort (consolidated standards of reporting trials) 2010 and spirit (standard protocol items:. It ensures consistency across clinical trial. The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human participants. Clinical trial protocol eudract number:. Welcome to global health trials' tools and templates library. The protocol is the backbone of your clinical trial, detailing every step of the study. The natural history/observational protocol template, the repository protocol template, and the secondary. The proposed guidance document includes a standardized template for manufacturers or other sponsors to use when developing ced study protocols using rwd. Phase 2 or 3 clinical trials that require. Trials is experimenting with a new way of structuring study protocols for randomised trials. Since the protocol and the clinical trial/study report are closely related, further relevant information can be found in the ich guideline for structure and content of clinical study. Clinical trial protocol eudract number:. Section headings and template text formatted in regular type should be included in your protocol document as provided in the template. Welcome to global health trials' tools. In this blog, you have access to the links to the clinical trial protocol template from several regulatory bodies. The proposed guidance document includes a standardized template for manufacturers or other sponsors to use when developing ced study protocols using rwd. Many recent studies have shown that highly selective biologic drugs are not effective in every patient and that variations. This report presents the explanation and elaboration paper for the consort (consolidated standards of reporting trials) 2010 and spirit (standard protocol items:. Developing a comprehensive clinical trial protocol. Trials is experimenting with a new way of structuring study protocols for randomised trials. Multicenter study to evaluate the efficacy and safety of secukinumab in controlling spinal pain in patients with axial. Developing a comprehensive clinical trial protocol. After reading, you will understand how to find a relevant clinical. It ensures consistency across clinical trial. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: Since the protocol and the clinical trial/study report are closely related, further relevant information can. There are three templates to be used for observational research: Describe and provide the results of animal studies, laboratory studies and pilot studies done in the usa or elsewhere, and clinical studies conducted abroad. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. Trials is experimenting. The goal of this template is to. Summarize the known and potential. After reading, you will understand how to find a relevant clinical. Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research study protocol specifically designed for the. There are three templates to be used for observational research: The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human participants. Clinical trial protocol cena713din01 / nct02989402 a prospective, 16 week, phase iv study to evaluate safety, tolerability and effectiveness in patients with severe dementia of Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate. Many recent studies have shown that highly selective biologic drugs are not effective in every patient and that variations in the genome can be associated with different clinical responses or. Section headings and template text formatted in regular type should be included in your protocol document as provided in the template. Nih applicants can use a template with instructional and. Since the protocol and the clinical trial/study report are closely related, further relevant information can be found in the ich guideline for structure and content of clinical study. Clinical trials are intended in their broadest sense and means any study design that involves an intervention regardless of the nature of that intervention (drug, biologic, device. It ensures consistency across clinical. Clinical trial protocol eudract number:. Many recent studies have shown that highly selective biologic drugs are not effective in every patient and that variations in the genome can be associated with different clinical responses or. Developing a comprehensive clinical trial protocol. The protocol is the backbone of your clinical trial, detailing every step of the study. Phase 2 or 3. There are three templates to be used for observational research: This report presents the explanation and elaboration paper for the consort (consolidated standards of reporting trials) 2010 and spirit (standard protocol items:. Many recent studies have shown that highly selective biologic drugs are not effective in every patient and that variations in the genome can be associated with different clinical responses or. Clinical trials are intended in their broadest sense and means any study design that involves an intervention regardless of the nature of that intervention (drug, biologic, device. Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research study protocol specifically designed for the. The natural history/observational protocol template, the repository protocol template, and the secondary. It ensures consistency across clinical trial. The template follows the international conference on harmonisation (ich) e6 (r2) good clinical practice and is available as a word document. The proposed guidance document includes a standardized template for manufacturers or other sponsors to use when developing ced study protocols using rwd. The simple innovation is to include all 51 spirit headings and item identifiers within the protocol. Developing a comprehensive clinical trial protocol. The goal of this template is to. Clinical trial protocol cena713din01 / nct02989402 a prospective, 16 week, phase iv study to evaluate safety, tolerability and effectiveness in patients with severe dementia of Welcome to global health trials' tools and templates library. Since the protocol and the clinical trial/study report are closely related, further relevant information can be found in the ich guideline for structure and content of clinical study. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:Clinical Trial Protocol Template Word
Free Clinical Trial Templates Smartsheet
Clinical Trial Protocol Synopsis Template
Clinical Study Protocol PowerPoint and Google Slides Template PPT Slides
Clinical Study Protocol Template
CLINICAL TRIAL PROTOCOL TEMPLATE DEVELOPING CLINICAL TRIAL
Clinical Study Protocol Template
Clinical Study Protocol (CSP) Template Clinical Study Templates
instructions for clinical research protocol template Doc Template
Clinical Study Protocol Template
Summarize The Known And Potential.
After Reading, You Will Understand How To Find A Relevant Clinical.
Describe And Provide The Results Of Animal Studies, Laboratory Studies And Pilot Studies Done In The Usa Or Elsewhere, And Clinical Studies Conducted Abroad.
Multicenter Study To Evaluate The Efficacy And Safety Of Secukinumab In Controlling Spinal Pain In Patients With Axial Spondyloarthritis Document Type:
Related Post: