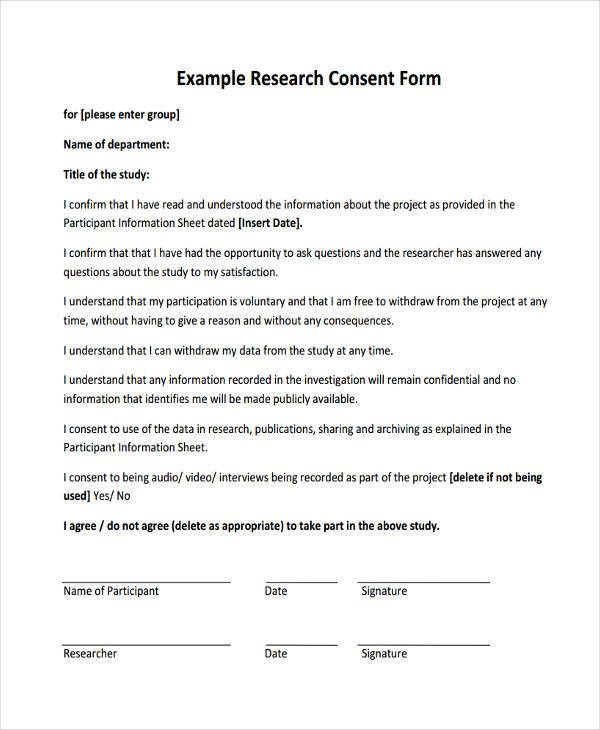
Consent Form Template For Research
Consent Form Template For Research - The templates on this page are intended to help investigators construct documents that are as short as possible and written in plain language. Please note that this is a template developed by the research ethics review office to assist research proponents in the design of their informed consent forms (icf). Don’t cut and paste from your. Include for studies that will place research information/consent form in the participant clinical/medical record(s) (required for studies involving treatment, care, or diagnosis): The consent template documents on this page include prompts for all the necessary elements of consent. Fill out the form online and save as pdf. See irb guidance on children and minors in research for information about the documentation needed for consenting children and parents, and use the sample consent and assent forms. Entifies consent elements or info. Ensure all necessary details, such as study objectives and. Using these templates to create your study documents will help to speed up the irb. See irb guidance on children and minors in research for information about the documentation needed for consenting children and parents, and use the sample consent and assent forms. Informed consent and hipaa authorization form template v09.2022a: Downloadable and printable, a consent form for research pdf offers convenience for researchers and participants alike. Download a free research consent form template. Protocol and consent (i.e., secondary research) will need to be met through other means. Include for studies that will place research information/consent form in the participant clinical/medical record(s) (required for studies involving treatment, care, or diagnosis): Short form consent templates can be found here. Please note that this is a template developed by the research ethics review office to assist research proponents in the design of their informed consent forms (icf). This consent should be used to obtain permission from subjects or the parent(s) of subjects (if minors will be. Learn about the changes to the regulations for informed consent and the irb submission. This template should be used as the consent document guide for all research studies, including parental and lar. Please note that this is a template developed by the research ethics review office to assist research proponents in the design of their informed consent forms (icf). Short form consent templates can be found here. It is important that principal. Customizable and. Include for studies that will place research information/consent form in the participant clinical/medical record(s) (required for studies involving treatment, care, or diagnosis): Identifies consent elements and information required for all st. Short form consent templates can be found here. Don’t cut and paste from your. A collection of informed consent, assent, and debriefing templates that can be used for your. Download our research consent form in pdf or ms word to ensure ethical compliance and informed participant agreement in your study. Ensure all necessary details, such as study objectives and. Investigators are required to use the latest versions of the informed consent form templates, which have been updated to comply with the 2019. Informed consent and hipaa authorization form template. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed consent forms (icf). Using these templates to create your study documents will help to speed up the irb. Informed consent and hipaa authorization form template v09.2022a: Download our research consent form in pdf or ms word to ensure. Investigators are required to use the latest versions of the informed consent form templates, which have been updated to comply with the 2019. This template can be used by researchers to gain informed consent to conduct research that collects data from people using questionnaires, observations, interviews, diaries, focus groups,. Don’t cut and paste from your. This template should be used. See irb guidance on children and minors in research for information about the documentation needed for consenting children and parents, and use the sample consent and assent forms. Customizable and ready to print. • modifications to approved protocols (need prior irb approval to implement). Include for studies that will place research information/consent form in the participant clinical/medical record(s) (required for. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. Customizable and ready to print. Required only when applicable to your study. Download a free research consent form template. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. Downloadable and printable, a consent form for research pdf offers convenience for researchers and participants alike. Required only when applicable to your study. Don’t cut and paste from your. • modifications to approved protocols (need prior irb approval to implement). This template can be used by researchers to gain informed consent to conduct research that collects data from people using. This template should be used as the consent document guide for all research studies, including parental and lar. Short form consent templates can be found here. Download our research consent form in pdf or ms word to ensure ethical compliance and informed participant agreement in your study. Include for studies that will place research information/consent form in the participant clinical/medical. Include for studies that will place research information/consent form in the participant clinical/medical record(s) (required for studies involving treatment, care, or diagnosis): Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed consent forms (icf). Please note that this is a template developed by the research ethics review. Identifies consent elements and information required for all st. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. Download our research consent form in pdf or ms word to ensure ethical compliance and informed participant agreement in your study. Find consent form templates and guidance for different types of research projects. • modifications to approved protocols (need prior irb approval to implement). See irb guidance on children and minors in research for information about the documentation needed for consenting children and parents, and use the sample consent and assent forms. The templates on this page are intended to help investigators construct documents that are as short as possible and written in plain language. Customizable and ready to print. This consent should be used to obtain permission from subjects or the parent(s) of subjects (if minors will be. Informed consent and hipaa authorization form template v09.2022a: This template can be used by researchers to gain informed consent to conduct research that collects data from people using questionnaires, observations, interviews, diaries, focus groups,. Include for studies that will place research information/consent form in the participant clinical/medical record(s) (required for studies involving treatment, care, or diagnosis): A research informed consent form is used to inform participants in a research study of how the research will be conducted, presented, and reported. Using these templates to create your study documents will help to speed up the irb. Ensure all necessary details, such as study objectives and. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed consent forms (icf).FREE 12+ Research Consent Form Samples & Templates
FREE 12+ Research Consent Form Samples & Templates
FREE 6+ Research Consent Forms in PDF MS Word
FREE 12+ Research Consent Form Samples & Templates
FREE 6+ Research Consent Forms in PDF MS Word
FREE 12+ Research Consent Form Samples & Templates
Free Research Informed Consent Form PDF Word eForms
Research Consent Form Fill Out, Sign Online and Download PDF
FREE 12+ Research Consent Form Samples, PDF, MS Word, Google Docs
FREE 12+ Research Consent Form Samples, PDF, MS Word, Google Docs
Downloadable And Printable, A Consent Form For Research Pdf Offers Convenience For Researchers And Participants Alike.
Required Only When Applicable To Your Study.
Please Note That This Is A Template Developed By The Research Ethics Review Office To Assist Research Proponents In The Design Of Their Informed Consent Forms (Icf).
Don’t Cut And Paste From Your.
Related Post: