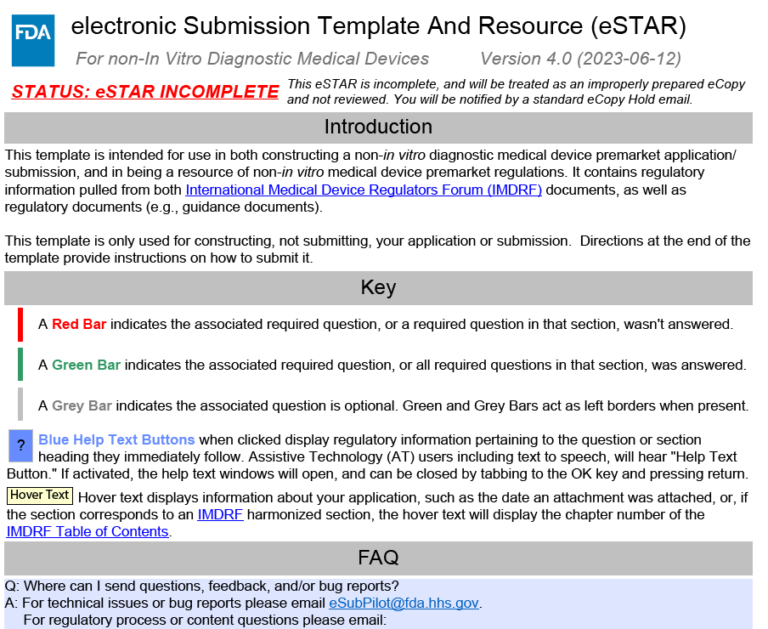
Fda Estar Template
Fda Estar Template - By using a standardized format, submitters can ensure completeness, and the fda can conduct reviews more efficiently, promoting timely access to safe and effective medical. The template provides a standard format for. Send medical device estar and ecopy premarket submissions online. The electronic submission template and resource (estar) program is a pivotal advancement in the regulatory landscape of medical device manufacturing. As of now, all 510k submissions must use the estar program. Mandatory use of the estar template for 510(k) submissions began in. Food and drug administration is announcing the voluntary electronic submission template and resource (estar) pilot program as an alternate method. The esubmitter template was developed by fda as an optional free tool consisting of a collection of questions, text, logic, and prompts that guides a user through preparation of a 510(k. Estar is designed to streamline the fda submission process, making it more efficient and standardized. The estar program enables medical device manufacturers to submit their approval documents to the fda via an interactive pdf template. The estar program, or electronic submission template and resource, is a program developed by the food and drug administration (fda) to provide a standardized electronic submission. The electronic submission template and resource (estar) program is a pivotal advancement in the regulatory landscape of medical device manufacturing. The united states food and drug administration (usfda) introduced the electronic submission template and resource (estar) program as part of its efforts to modernize and. Find out the benefits, challenges, and updates of the. The estar template became available for voluntary use by all 510(k) submitters in september 2020. Send medical device estar and ecopy premarket submissions online. The electronic submission template and resource (estar) is an interactive pdf form that guides applicants through the process of preparing a comprehensive medical device. Fda is partnering with health canada to launch a joint electronic submission template and resource (estar) pilot with the intent of allowing sponsors to. As of now, all 510k submissions must use the estar program. Estar is designed to streamline the fda submission process, making it more efficient and standardized. Fda is partnering with health canada to launch a joint electronic submission template and resource (estar) pilot with the intent of allowing sponsors to. What is the estar program? As of now, all 510k submissions must use the estar program. The united states food and drug administration (usfda) introduced the electronic submission template and resource (estar) program as part of. Fda is partnering with health canada to launch a joint electronic submission template and resource (estar) pilot with the intent of allowing sponsors to. The estar program enables medical device manufacturers to submit their approval documents to the fda via an interactive pdf template. Send medical device estar and ecopy premarket submissions online. The electronic submission template and resource (estar). The electronic submission template and resource (estar) program is a pivotal advancement in the regulatory landscape of medical device manufacturing. What is the estar program? By using a standardized format, submitters can ensure completeness, and the fda can conduct reviews more efficiently, promoting timely access to safe and effective medical. The united states food and drug administration (usfda) introduced the. The united states food and drug administration (usfda) introduced the electronic submission template and resource (estar) program as part of its efforts to modernize and. Learn how to use fda's estar template for 510 (k) premarket notifications, which is required since october 1, 2023. Fda is partnering with health canada to launch a joint electronic submission template and resource (estar). The template provides a standard format for. As of now, all 510k submissions must use the estar program. The estar program, or electronic submission template and resource, is a program developed by the food and drug administration (fda) to provide a standardized electronic submission. What is the estar program? The fda's 510(k) estar template is a powerful tool that can. The estar program enables medical device manufacturers to submit their approval documents to the fda via an interactive pdf template. The esubmitter template was developed by fda as an optional free tool consisting of a collection of questions, text, logic, and prompts that guides a user through preparation of a 510(k. The electronic submission template and resource (estar) program is. The estar program, or electronic submission template and resource, is a program developed by the food and drug administration (fda) to provide a standardized electronic submission. As of now, all 510k submissions must use the estar program. The estar template became available for voluntary use by all 510(k) submitters in september 2020. The template provides a standard format for. The. The fda's 510(k) estar template is a powerful tool that can help you streamline the process of submitting a 510(k) notification. Food and drug administration is announcing the voluntary electronic submission template and resource (estar) pilot program as an alternate method. Mandatory use of the estar template for 510(k) submissions began in. The esubmitter template was developed by fda as. Mandatory use of the estar template for 510(k) submissions began in. Learn how to use fda's estar template for 510 (k) premarket notifications, which is required since october 1, 2023. By using a standardized format, submitters can ensure completeness, and the fda can conduct reviews more efficiently, promoting timely access to safe and effective medical. The united states food and. The template provides a standard format for. Estar is designed to streamline the fda submission process, making it more efficient and standardized. Food and drug administration is announcing the voluntary electronic submission template and resource (estar) pilot program as an alternate method. Send medical device estar and ecopy premarket submissions online. As of now, all 510k submissions must use the. Learn how to use fda's estar template for 510 (k) premarket notifications, which is required since october 1, 2023. What is the estar program? The fda's 510(k) estar template is a powerful tool that can help you streamline the process of submitting a 510(k) notification. Food and drug administration is announcing the voluntary electronic submission template and resource (estar) pilot program as an alternate method. The template provides a standard format for. The electronic submission template and resource (estar) is an interactive pdf form that guides applicants through the process of preparing a comprehensive medical device. The estar program enables medical device manufacturers to submit their approval documents to the fda via an interactive pdf template. Estar is designed to streamline the fda submission process, making it more efficient and standardized. The electronic submission template and resource (estar) program is a pivotal advancement in the regulatory landscape of medical device manufacturing. Mandatory use of the estar template for 510(k) submissions began in. Fda is partnering with health canada to launch a joint electronic submission template and resource (estar) pilot with the intent of allowing sponsors to. As of now, all 510k submissions must use the estar program. The estar program, or electronic submission template and resource, is a program developed by the food and drug administration (fda) to provide a standardized electronic submission. The esubmitter template was developed by fda as an optional free tool consisting of a collection of questions, text, logic, and prompts that guides a user through preparation of a 510(k. By using a standardized format, submitters can ensure completeness, and the fda can conduct reviews more efficiently, promoting timely access to safe and effective medical.Das eStarProgramm der FDA Pflicht oder Chance?
About U.S FDA eSTAR eStarHelper
What is the FDA eSTAR program?
FDA eSTAR Submission Template A Guide to Navigating the Process
FDA 510k eSTAR submissions with 100 acceptance Essenvia
Using the new eSTAR templates for a 510(k) submission and the FDA eSTAR
FDA 510(k) Submissions to Use eSTAR Change in Submissions
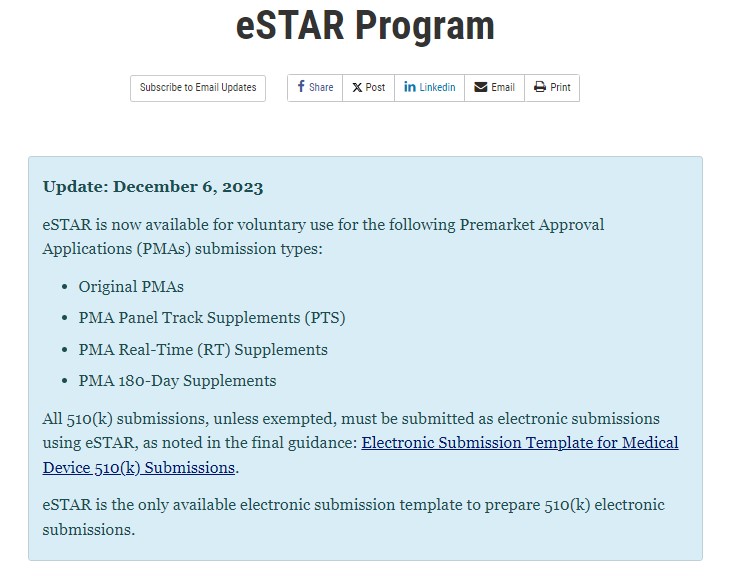
FDA updates their eSTAR templates to submit certain PMA applications
FDA eSTAR Submission Template A Guide to Navigating the Process
About U.S FDA eSTAR eStarHelper
Send Medical Device Estar And Ecopy Premarket Submissions Online.
Find Out The Benefits, Challenges, And Updates Of The.
The Estar Template Became Available For Voluntary Use By All 510(K) Submitters In September 2020.
The United States Food And Drug Administration (Usfda) Introduced The Electronic Submission Template And Resource (Estar) Program As Part Of Its Efforts To Modernize And.
Related Post:
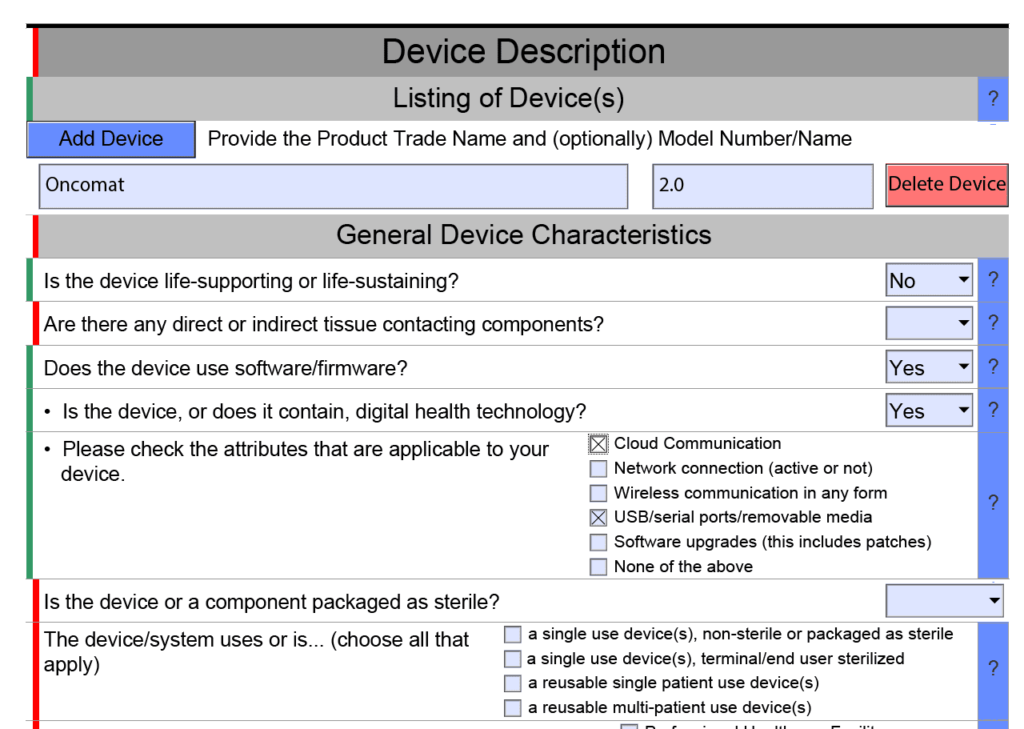

.jpg)