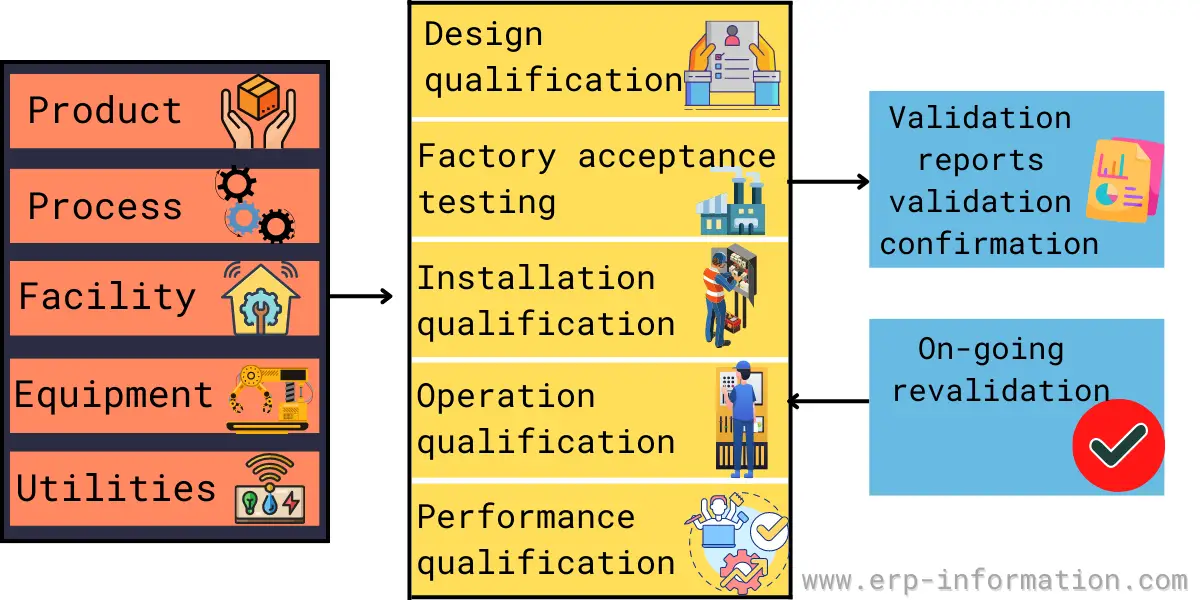
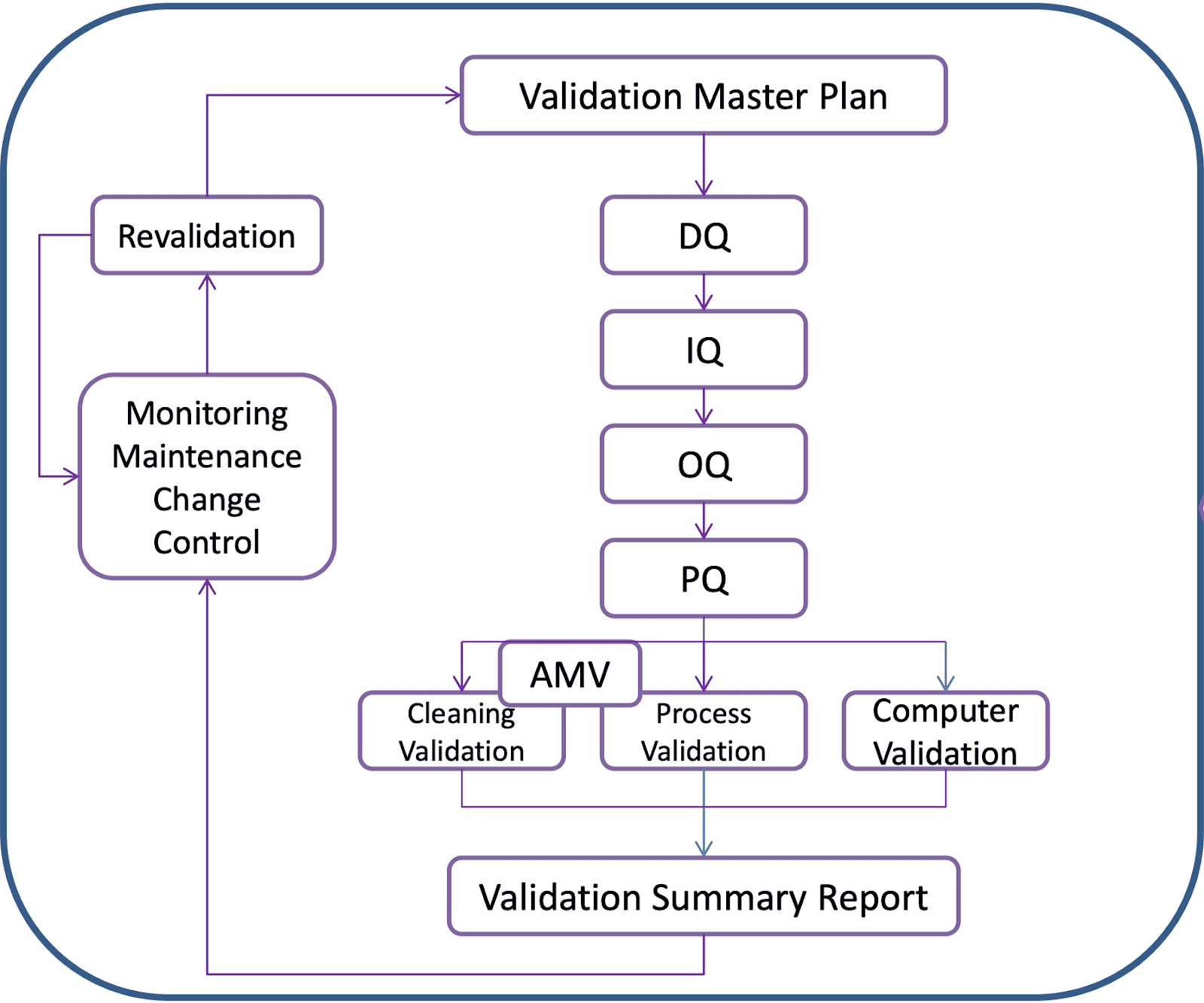
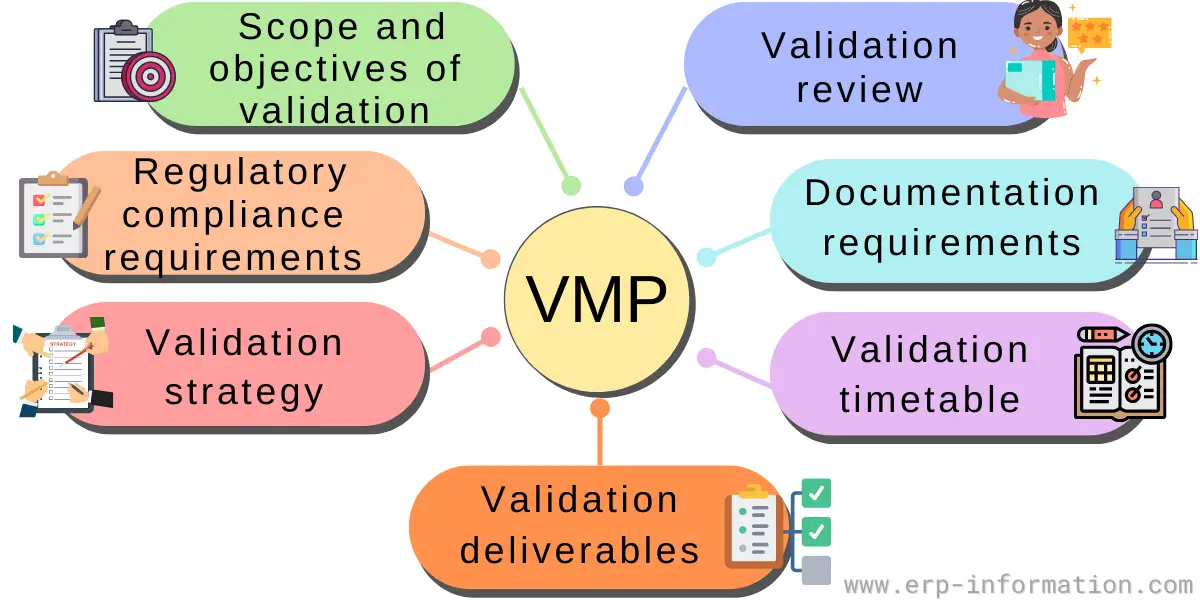
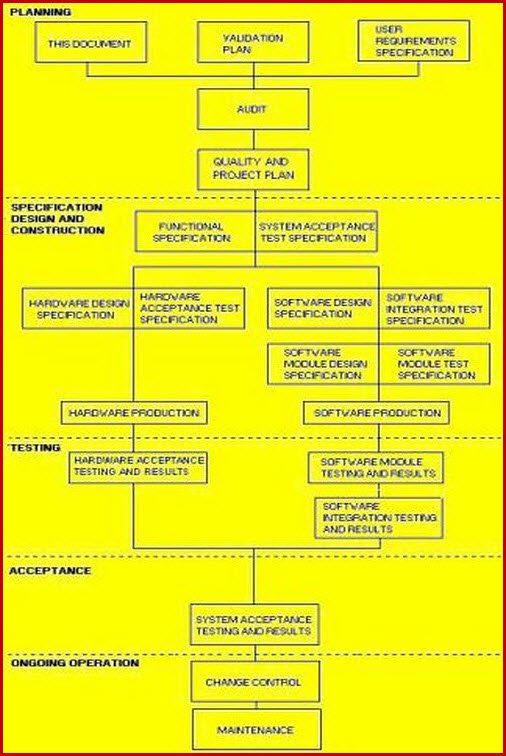
Vmp Template
Vmp Template - This document outlines the validation master plan for acme. This protocol template provides a comprehensive validation master plan (vmp) protocol for pharmaceutical and medical device companies. Validation document template is available at site, however additional contents can be included wherever deemed necessary. Facilities and utilities, equipment, processes, and computer systems. Different major projects carried out in one facility may each have. It covers the validation of facilities, equipment, processes,. It outlines a structured approach for. Validation master plan (vmp) the vmp serves as the validation roadmap, setting the course, justifying the strategy, out lining the preliminary test and acceptance criteria, and documenting. What is validation master plan (vmp): This article can help you understand the principle of a. The purpose of the validation master plan is to document the compliance requirements for the site and to ensure that. It outlines a structured approach for. Template helps to maintain the consistency and uniformity. The vmp is a useful aid for mhra inspectors and other auditors in understanding what validation activities have been performed and the unit’s approach to validation. You might wonder what a master validation plan (mvp) is and how to develop and implement one for your gmp facility. The requirements for specific validation activities will be defined in guidelines and procedures. The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and acceptance. This validation master plan (vmp) documents the general approach to validation at site, site. This document outlines the validation master plan for acme. It covers the validation of facilities, equipment, processes,. The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and acceptance. You might wonder what a master validation plan (mvp) is and how to develop and implement one for your gmp facility. What is validation master plan (vmp): A validation master plan (also referred to as the vmp) is. Different major projects carried out in one facility may each have. You might wonder what a master validation plan (mvp) is and how to develop and implement one for your gmp facility. This protocol template provides a comprehensive validation master plan (vmp) protocol for pharmaceutical and medical device companies. The validation master plan is a summary of validation strategy. This. Template helps to maintain the consistency and uniformity. This validation master plan (vmp) describes the validation requirements for a manufacturing site located at a specific address. You might wonder what a master validation plan (mvp) is and how to develop and implement one for your gmp facility. The requirements for specific validation activities will be defined in guidelines and procedures.. The purpose of the validation master plan is to document the compliance requirements for the site and to ensure that. The validation master plan is a summary of validation strategy. What is validation master plan (vmp): This document outlines the validation master plan for acme. Facilities and utilities, equipment, processes, and computer systems. Validation document template is available at site, however additional contents can be included wherever deemed necessary. The validation master plan is a summary of validation strategy. This document outlines the validation master plan for acme. Where a project consists of a range of different validation activities then a validation master plan (vmp) should be prepared. This article can help you. The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and acceptance. The vmp is a useful aid for mhra inspectors and other auditors in understanding what validation activities have been performed and the unit’s approach to validation. Different major projects carried out in one facility may each have. This. Different major projects carried out in one facility may each have. It covers the validation of facilities, equipment, processes,. The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and acceptance. This validation master plan (vmp) documents the general approach to validation at site, site. What is validation master plan. This article can help you understand the principle of a. Template helps to maintain the consistency and uniformity. The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and acceptance. What is validation master plan (vmp): The purpose of the validation master plan is to document the compliance requirements for. The requirements for specific validation activities will be defined in guidelines and procedures. This validation master plan (vmp) documents the general approach to validation at site, site. The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and acceptance. The validation master plan is a summary of validation strategy. The. Give the location of the facility and define the types of validations that are included: Validation master plan (vmp) the vmp serves as the validation roadmap, setting the course, justifying the strategy, out lining the preliminary test and acceptance criteria, and documenting. Different major projects carried out in one facility may each have. Facilities and utilities, equipment, processes, and computer. The requirements for specific validation activities will be defined in guidelines and procedures. This document outlines the validation master plan for acme. It covers the validation of facilities, equipment, processes,. Where a project consists of a range of different validation activities then a validation master plan (vmp) should be prepared. The vmp is a useful aid for mhra inspectors and other auditors in understanding what validation activities have been performed and the unit’s approach to validation. Template helps to maintain the consistency and uniformity. Give the location of the facility and define the types of validations that are included: The validation master plan is a summary of validation strategy. This article can help you understand the principle of a. What is validation master plan (vmp): You might wonder what a master validation plan (mvp) is and how to develop and implement one for your gmp facility. Facilities and utilities, equipment, processes, and computer systems. The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and acceptance. This validation master plan (vmp) documents the general approach to validation at site, site. Validation master plan (vmp) the vmp serves as the validation roadmap, setting the course, justifying the strategy, out lining the preliminary test and acceptance criteria, and documenting. A validation master plan (also referred to as the vmp) is a document which outlines the principles tied to the qualification of a certain facility, defining the systems and.What is Validation Master Plan? (Template, Examples)
What is a Validation Master Plan (VMP)? GetReskilled
Validation Master Plan Template Printable Calendars AT A GLANCE
VMP Template PDF Verification And Validation Business Process
What is Validation Master Plan? (Template, Examples)
Validation Master Plan (VMP) Downloadable Interactive Template.
Validation Master Plan (VMP) Template LexDocPharma
Image Validation Master Plan TEMPLATE (Technical Series on Process
Validation Master Plan Template
Validation Master Plan Template
Different Major Projects Carried Out In One Facility May Each Have.
This Protocol Template Provides A Comprehensive Validation Master Plan (Vmp) Protocol For Pharmaceutical And Medical Device Companies.
The Purpose Of The Validation Master Plan Is To Document The Compliance Requirements For The Site And To Ensure That.
Validation Document Template Is Available At Site, However Additional Contents Can Be Included Wherever Deemed Necessary.
Related Post: